How Kamal-Sourour parameters in Kinetics Neo correspond to original equation da/dt=(k1+k2*a^m)*(1-a)^n?
Kamal-Sourour model in the literature [1] is developed for curing of epoxy at isothermal conditions and therefore does not contain the temperature dependence for the rate constants:
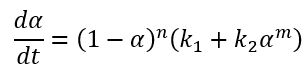
In Kinetics Neo we use extended, improved version of equation, where the reaction rates have the Arrhenius dependence on temperature:
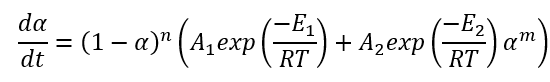
If the pre-exponential factor A1 is placed outside of brackets, then the equation looks as following:
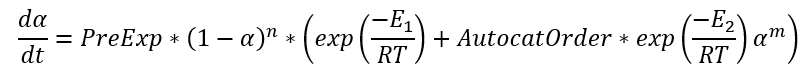
where PreExp = A1, AutocatOrder = A2/A1
For reaction A→B the reactant A has concentration a (changes from 1 to 0 during reaction), and the product B has concentration b (changes from 0 to 1). Degree of conversion alpha corresponds to concentration b of product B, and (1- alpha) corresponds to concentration a of reactant A. After this replacement the kinetic equation is rewritten using concentrations (here is example for step A→B):
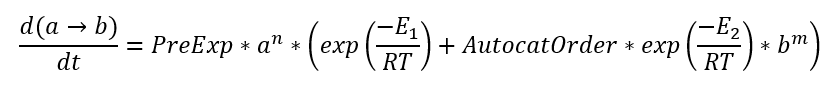
This equation is equal to equation in Kinetics Neo:
d(a->b)/dt=PreExp*a^n* [Exp(-ActivationEnergy/(RT)) +AutocatOrder*(b^m)*Exp(-ActEnergy2/(RT))]
where ActivationEnergy = E1, and ActEnergy2=E2.
For isothermal conditions this equation is equal to classical Kamal-Sourour equation:
dα/dt=(k1+k2*αm)*(1-α)n with k1= PreExp* Exp(-ActivationEnergy/(RT)) and k2= PreExp* AutocatOrder *Exp(-ActivationEnergy/(RT))
Simplified Kamal-Sourour models in Kinetics Neo:
Cmn is the simplified Kamal-Sourour reaction, where E1=E2
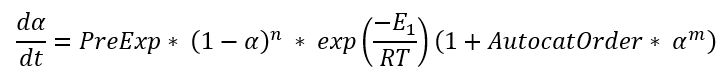
Cn is the simplified Kamal-Sourour reaction, where E1=E2and m=1
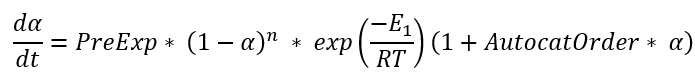
C1 is the simplified Kamal-Sourour reaction, where E1=E2 m=1 and n=1
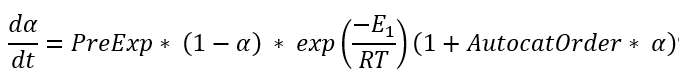
Referencees
1. S.Sourour, M.R.Kamal Differential Scanning Calorimetry of Epoxy Cure: Isothermal Cure Kinetics, Thermochimica Acta, 14 (1976) 41-59.
